Chemistry
A knowledge of basic chemistry is important for understanding just about any area of biology from the function of cells to the behavior of organisms and the ecological relationships between organisms and their environment.
Indeed, chemists are fond of teasing biologists by claiming that all biology is chemistry*. While this isn't quite true, in order to make sense of the structure of cells and organisms a little chemistry goes a long way.
Atoms are basic components of the matter we are familiar with. Atoms are the smallest part of matter that have chemical properties characteristic of a particular chemical element. Most of the mass of an atom is due to the atomic nucleus. The nucleus consists of protons which have a positive electrical charge and neutrons which have no charge. This is a representation of a carbon atom.
Atomic number and atomic mass. The atomic number of an atom is the number of protons in the atom which, if the atom is neutral, equals the number of electrons. This is important because the number of electrons is related to the chemical properties of the atom. The atomic mass is the total mass of the electrons, protons, neutrons in the atom.
Isotopes are atoms that have the same atomic number(number of protons) but differ in atomic mass. For instance most carbon has six protons and six neutrons and thus atomic mass of of approxiately 12. Some carbon atoms have an atomic mass of 13 so thus have six protons and seven neutrons. All isotopes of the same element have about the same chemical properites because the number pattern of electrons is the same.
Radio-isotopes. Many elements have multiple isotopes, some of which may ne radioactive. Radioactive isotopes are called radio-isotopes and are characterized by a constant rate of decay into other elements or isotopes. Some common isotopes in biology and their half lives are here.
The half life of a radio-isotope is the time in which it takes half of a starting amount of radio-isotope to decay into something else. This decay is accompanied by the release of various forms of radiation. This is important for biologists because it allows biologists to use radioisotopes to trace the fate of elements in biological systems and to date fossils by measuring the ratios of different radio-isotopes in the fossil.
Electrons are small components which are surrounding the nucleus. Electron have a negative charge. An atom with no net electrical charge has the same number of electrons as protons. Electrons are restricted to certain energy levels "shells" and orbitals within those energy levels and these energy levels and orbitals fill in a regular pattern from the lowest energy level outward. Here is an illustration of the orbitals of a carbon atom's outer energy level. Orbital is in some sense a bad word because electrons tend to behave rather strangely in that its orbit is best described as a probability density.
Solids, Liquids, Gases Compared
Solids
The particles of a solid are always arranged in an orderly manner. They have a constant volume, because the particles are so closely packed together, with very little space between them. Compression of a solid to any large extent is not possible because of this tight pack of particles.Liquids
A fluid is any substance that flows, and liquids are examples of fluids. The particles in liquids are allowed to freely move and change their positions. At all times are the particles moving, moving from neighbor to neighbor. This is why we can 'pour' a liquid into another container. A liquids confinement are the borders of its container. This is why when we pour a liquid into another container, there is conformity to the shape of the container. Compression of a liquid to any large extent is not possible.Gases
Gases is another example of a fluid, it flows! The particles of gases are however much different than that of solids and liquids. The particles in gases are not neatly arranged, and they don't even touch each other most of the time. There is lots of space in between particles, which is why when put in a container, it is filled with the gas. And when released from a container, the gas is dispersed. The particles in gases are always moving, just like the particles in a liquid. Electrons, Chemical Bonds and Periodic Table.
The chemical properties of an atom are determined largely by how full or empty the outer electron shell is. For example, atoms of fluorine(F), chlorine(Cl) and the other elements in that second from the last column of the periodic table need only one electron to fill the outer shell. These atoms have a very strong tendency to steal electrons from other atoms. Oxygen and sulphur have 6 electrons in their outer shell which again holds 8 maximum. Thus these elements tend to steal electrons.
Elements such as Lithium(Li) Sodium(Na) and Potassium(K) on the left hand side of the periodic table have an almost empty shell and these elements readily give up those outer shell electrons to atoms such as oxygen and chlorine. Elements that tend to give up electrons to other atoms are called metals.Elements in the middle of the periodic table tend to share electrons rather than give them up or take them entirely. Many of these such as iron, copper or gold are also considered metals.
The elements at the far right: Helium, Neon, Argon etc... are chemically inert because they have a full outer shell. They will only react with other chemicals under very special conditions. These elements are sometimes called the 'noble' or inert gases because it is so difficult to get them to form chemical bonds.
Chemical Bonds.
A chemical bond is an attraction between atoms brought about by a sharing of electrons between to atoms or a complete transfer of electrons. There are three types of chemical bonds: Ionic, Covalent and Polar covalent. In addition chemists often recognize another type of bond called a hydrogen bond.
Ionic Bonds |
A well known example is table salt, sodium chloride. Sodium gives up its one outer shell electron completely to chlorine which needs only one electron to fill its shell. Thus, the attraction between these atoms is much like static electricity since opposite charges attract.
Ionic, Covalent, Polar covalent.
Covalent bond.Covalent bonds involve a complete sharing of electrons and occurrs most commonly between atoms that have partially filled outer shells or energy levels. Thus if the atoms are similar in negativity then the electrons will be shared. Carbon forms covalent bonds. The electrons are in hybrid orbitals formed by the atoms involved as in this example: ethane. Diamond is strong because it involves a vast network of covalent bonds between the carbon atoms in the diamond.
Polar Covalent Bond. These bonds are in between covalent and ionic bonds in that the atoms share electrons but the electrons spend more of their time around on atom versus the others in the compound. This type of bond occurs when the atoms involved differ greatly in electronegative. The most familiar example is water. Oxygen is much more electronegative than hydrogen, and so the electrons involved in bonding the water molecule spend more time there. The fact that water is a polar covalently bonded molecule has a number of implications for molecules that are dissolved in water. In particular, molecules with polar covalent bods can break apart when they encounter water molecules. They are broken apart because of the electrical attraction between the dissimilar charges of the molecules. Also, since ironically bonded molecules involve ions with opposite charges, water with its polar covalent bonds can separate ions from each other and then surround the ions which prevents them from recombining. The properties of water all relate to this polar covalent bonding. Indeed the sorts of so called hydrophobic and hydrophobic interactions water has with various organic compounds depend on the nature of the polar covalent bond in water.
Hydrogen Bond.The fact that the oxygen end of a water molecule is negatively charged and the hydrogen end positively charged means that the hydrogen of one water molecule attract the oxygen of its neighbor and vice verse. This is because unlike charges attract. This largely electrostatic attraction is called a hydrogen bond and is important in determining many important properties of water that make it such an important liquid for living things. Water can also form this type of bond with other polar molecules or ions such as hydrogen or sodium ions. Further, hydrogen bonds can occurr within and between other molecules. For instance, the two strands of a DNA molecule are held together by hydrogen bonds. Hydrogen bonding between water molecules and the amino acids of proteins are involved in maintaining the protein's proper shape.
The pH Scale.
PHYSICES
Mechanics and Motion
 Motion is one of the key topics in physics. Everything in the universe moves. It might only be a small amount of movement and very very slow, but movement does happen. Don't forget that even if you appear to be standing still, the Earth is moving around the Sun, and the Sun is moving around our galaxy. The movement never stops. Motion is one part of what physicists call mechanics. Over the years, scientists have discovered several rules or laws that explain motion and the causes of changes in motion. There are also special laws when you reach the speed of light or when physicists look at very small things like atoms.
Motion is one of the key topics in physics. Everything in the universe moves. It might only be a small amount of movement and very very slow, but movement does happen. Don't forget that even if you appear to be standing still, the Earth is moving around the Sun, and the Sun is moving around our galaxy. The movement never stops. Motion is one part of what physicists call mechanics. Over the years, scientists have discovered several rules or laws that explain motion and the causes of changes in motion. There are also special laws when you reach the speed of light or when physicists look at very small things like atoms. Speed it Up, Slow it Down
The physics of motion is all about forces. Forces need to act upon an object to get it moving, or to change its motion. Changes in motion won't just happen on their own. So how is all of this motion measured? Physicists use some basic terms when they look at motion. How fast an object moves, its speed or Velocity, can be influenced by forces. (Note: Even though the terms 'speed' and 'velocity' are often used at the same time, they actually have different meanings.) Acceleration is a twist on the idea of velocity. Acceleration is a measure of how much the velocity of an object changes in a certain time (usually in one second). Velocities could either increase or decrease over time. Mass is another big idea in motion. Mass is the amount of something there is, and is measured in grams (or kilograms). A car has a greater mass than a baseball.
Acceleration is a twist on the idea of velocity. Acceleration is a measure of how much the velocity of an object changes in a certain time (usually in one second). Velocities could either increase or decrease over time. Mass is another big idea in motion. Mass is the amount of something there is, and is measured in grams (or kilograms). A car has a greater mass than a baseball. Simple and Complex Movement
There are two main ideas when you study mechanics. The first idea is that there are simple movements, such as if you're moving in a straight line, or if two objects are moving towards each other in a straight line. The simplest movement would be objects moving at constant velocity. Slightly more complicated studies would look at objects that speed up or slow down, where forces have to be acting.There are also more complex movements when an object's direction is changing. These would involve curved movements such as circular motion, or the motion of a ball being thrown through the air. For such complex motions to occur, forces must also be acting, but at angles to the movement.
In order to really understand motion, you have to think about forces, acceleration, energy, work, and mass. These are all a part of mechanics.
Heat and Thermal Energy
When scientists originally studied thermodynamics, they were really studying heat and thermal energy. Heat can do anything: move from one area to another, get atoms excited, and even increase energy. Did we say energy? That's what heat is. When you increase the heat in a system, you are really increasing the amount of energy in the system. Now that you understand that fact, you can see that the study of thermodynamics is the study of the amount of energy moving in and out of systems.Heat of Atoms
Now all of this energy is moving around the world. You need to remember that it all happens on a really small scale. Energy that is transferred is at an atomic level. Atoms and molecules are transmitting these tiny amounts of energy. When heat moves from one area to another, it's because millions of atoms and molecules are working together. Those millions of pieces become the energy flow throughout the entire planet.Heat Movement
Heat moves from one system to another because of differences in the temperatures of the systems. If you have two identical systems with equal temperatures, there will be no flow of energy. When you have two systems with different temperatures, the energy will start to flow. Air mass of high pressure forces large numbers of molecules into areas of low pressure. Areas of high temperature give off energy to areas with lower temperature. There is a constant flow of energy throughout the universe. Heat is only one type of that energy.Increasing Energy and Entropy
Another big idea in thermodynamics is the concept of energy that changes the freedom of molecules. For example, when you change the state of a system (solid, liquid, gas), the atoms and/or molecules have different arrangements and degrees of freedom to move. That increase in freedom is called entropy. Atoms are able to move around more and there is more activity. That increase in freedom (also called randomness) is an increase in entropy.
Moving Electrons and Charges
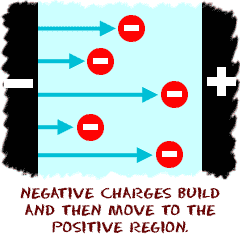 Electricity is related to charges, and both electrons and protons carry a charge. The amount of the charge is the same for each particle, but opposite in sign. Electrons carry a negative charge while protons carry positive charge. The objects around us contain billions and billions of atoms, and each atom contains many protons and electrons. The protons are located in the center of the atom, concentrated in a small area called the nucleus. The electrons are in motion outside of the nucleus in orbitals. The protons are basically trapped inside the nucleus and can't escape the nucleus. As a result, it is moving electrons that are primarily responsible for electricity.
Electricity is related to charges, and both electrons and protons carry a charge. The amount of the charge is the same for each particle, but opposite in sign. Electrons carry a negative charge while protons carry positive charge. The objects around us contain billions and billions of atoms, and each atom contains many protons and electrons. The protons are located in the center of the atom, concentrated in a small area called the nucleus. The electrons are in motion outside of the nucleus in orbitals. The protons are basically trapped inside the nucleus and can't escape the nucleus. As a result, it is moving electrons that are primarily responsible for electricity. There aren't a lot of places that you can see electricity. The most commonly- observed form of electricity is probably lightning. Lightning is a big spark that occurs when lots of electrons move from one place to another very quickly. There are three basic forms of lightning, cloud to cloud, cloud to surface, and surface to cloud. All are created when there is an unequal distribution of electrons. You can also see smaller sparks of electricity in science labs that contain Van de Graff generators, and can see even smaller arcs of electrons at home when you scuff your feet and then touch something like a metal doorknob (static electricity).
Electricity Around You
It's easy to see the uses of electricity around you. In fact, there are charges around your computer, your house, and your city. Electricity is constantly flowing through all of the wires in your town. There is also electricity in your flash light. That kind of electricity created by batteries is called direct current. The other major type is found in the outlets of your house. That household form of electricity is called alternating current.
Types of Light
To understand light you have to know that what we call light is what is visible to us. Visible light is the light that humans can see. Other animals can see different types of light. Dogs can see only shades of gray and some insects can see light from the ultraviolet part of the spectrum. The key thing to remember is that our light is what scientists call visible light.Scientists also call light electromagnetic radiation. Visible light is only one small portion of a family of waves called electromagnetic (EM) radiation. The entire spectrum of these EM waves includes radio waves, which have very long wavelengths and both gamma rays and cosmic rays, which are at the other end of the spectrum and have very small wavelengths. Visible light is near the middle of the spectrum.
It's all Energy
The key thing to remember is that light and EM radiation carry energy. The quantum theory suggests that light consists of very small bundles of energy/particles; it's just that simple. Scientists call those small particles photons, and the wavelength determines the energy and type of EM radiation, and the number of photons tells you how much radiation there is. A lot of photons give a brighter, more intense type of light. Fewer photons give a very dim and less intense light. When you use the dimmer switch on the wall, you are decreasing the number of photons sent from the light bulb. The type of light is the same while the amount has changed.Different Speeds of Light?
As far as we know, all types of light move at one speed when in a vacuum. The speed of light in a vacuum is 299,792,458 meters per second. That speed is really fast, but even when you're traveling that fast, it takes a while to get places in space. It takes about seven minutes for light from the Sun to reach Earth. It takes over four years for the light from our Sun to get to the nearest star. It would take a particle of light over 100,000 years to get from one side of our galaxy to the other side. All of those values are light moving through a vacuum. You can slow light down in other substances such as the atmosphere, water, or a diamond. Light moves at about 124,000,000 meters per second (less than half the speed in a vacuum) in a diamond.
Where Traditional Physics Stops
We're about to move into the modern age of physics. In the early 1800's, scientists began examining the basis of matter, space, and time. Sometimes it gets very confusing, but the big idea is that Newton's physics describe about 90% of the way things work in the universe (mechanics). His ideas start to break down when you talk about ideas such as objects moving at the speed of light, the inside of atoms, extreme temperatures, and when the objects are huge (like galaxies interacting with each other).Into the Atom
The original idea of atoms developed by Niels Bohr showed a structure based on various shells and a center area called the nucleus. The electrons were found in those shells while the protons and neutrons were found in the nucleus. There are other ways to look at the structure of atoms (you may have heard of "spdf"), but we're going to stick with the classic view for many of our discussions. This view of the structure of an atom was one of the foundations for modern physics.Into the Universe
Albert Einstein also played a large part in modern physics. He developed formulas that described the way matter and energy were related. Just about everyone has heard of the formula E=mc^2. That formula explains how energy is related to mass. The idea found its way into the study of fission reactions, and it was proved that enormous amounts of energy were stored in even one atom of a substance.Current Studies
Even now, scientists are still testing the boundaries of physics and the laws of physics. Only a few years ago a new state of matter was created. The Bose-Einstein condensate was theorized decades ago, but scientists have only recently been able to create it in a lab. Every day astronomers are studying space and learning how black holes and galaxies interact. Stephen Hawking is one of the more famous scientists working in that field. Our point is, there is still much to discover.Physics Formulas
Motion in One Dimension
The physics formulas for motion in one dimension (Also called Kinematical equations of motion) are as follows. (Here 'u' is initial velocity, 'v' is final velocity, 'a' is acceleration and t is time):
- s = ut + ½ at2
- v = u + at
- v2 = u2 + 2as
- vav(Average Velocity) = (v+u)/2
Physics Formulas for momentum, impulse and force concerning a particle moving in 3 dimensions are as follows (Here force, momentum and velocity are vectors ):
- Momentum is the product of mass and velocity of a body. Momentum is calculate using the formula: P = m (mass) x v (velocity)
- Force can defined as something which causes a change in momentum of a body. Force is given by the celebrated newton's law of motion: F = m (mass) x a (acceleration)
- Impulse is a large force applied in a very short time period. The strike of a hammer is an impulse. Impulse is given by I = m(v-u)
Pressure is defined as force per unit area:
| Pressure (P) = | Force (F) Force (A) |
Density
Density is the mass contained in a body per unit volume.
The physics formula for density is:
| Density (D) = | Mass(M) Volume (V) |
Angular Momentum
Angular momentum is an analogous quantity to linear momentum in which the body is undergoing rotational motion. The physics formula for angular momentum (J) is given by:
J = r x p
where J denotes angular momentum, r is radius vector and p is linear momentum.
Torque
Torque can be defined as moment of force. Torque causes rotational motion. The formula for torque is: τ = r x F, where τ is torque, r is the radius vector and F is linear force.
Circular Motion
The physics formulas for circular motion of an object of mass 'm' moving in a circle of radius 'r' at a tangential velocity 'v' are as follows:
| Centripetal force (F) = | mv2 r |
| Centripetal Acceleration (a) = | v2 r |
Center of Mass
General Formula for Center of mass of a rigid body is :
| R = | ΣNi = 1 miri ΣNi = 1mi |
where R is the position vector for center of mass, r is the generic position vector for all the particles of the object and N is the total number of particles.
Reduced Mass for two Interacting Bodies
The physics formula for reduced mass (μ) is :
| μ = | m1m2 m1 + m2 |
Work and Energy
Physics formulas for work and energy in case of one dimensional motion are as follows:
W (Work Done) = F (Force) x D (Displacement)
Energy can be broadly classified into two types, Potential Energy and Kinetic Energy. In case of gravitational force, the potential energy is given by
P.E.(Gravitational) = m (Mass) x g (Acceleration due to Gravity) x h (Height)
The transitional kinetic energy is given by ½ m (mass) x v2(velocity squared)
Power
Power is, work done per unit time. The formula for power is given as
| Power (P) = | V2 R | =I2R |
Newtonian Gravity
Here are some important physics formulas related to Newtonian Gravity:
Newton's Law of universal Gravitation:
| Fg = | Gm1m2 r2 |
- m1, m2 are the masses of two bodies
- G is the universal gravitational constant which has a value of 6.67300 × 10-11 m3 kg-1 s-2
- r is distance between the two bodies
- M is mass of central gravitating body
- R is radius of the central body
Here are two important physics formulas related to projectile motion:
(v = velocity of particle, v0 = initial velocity, g is acceleration due to gravity, θ is angle of projection, h is maximum height and l is the range of the projectile.)
| Maximum height of projectile (h) = | v0 2sin2θ 2g |
Horizontal range of projectile (l) = v0 2sin 2θ / g
Simple Pendulum
The physics formula for the period of a simple pendulum (T) = 2π √(l/g)where
- l is the length of the pendulum
- g is acceleration due to gravity
The Period of a conical pendulum (T) = 2π √(lcosθ/g)
where
- l is the length of the pendulum
- g is acceleration due to gravity
- Half angle of the conical pendulum
Here are some physics formulas related to electricity.
Resistance
The physics formulas for equivalent resistance in case of parallel and series combination are as follows:
Resistances R1, R2, R3 in series:
Req = R1 + R2 + R3
Resistances R1, R2, R3 in parallel:
| Req = | R1R2R3 R1 + R2 + R3 |
Ohm's Law
Ohm's law gives a relation between the voltage applied an current flowing across a solid conductor:
V (Voltage) = I (Current) x R (Resistance)
Power
In case of a closed electrical circuit with applied voltage V and resistance R, through which current I is flowing,
| Power (P) = | V2 R |
= I2R. . . (because V = IR, Ohm's Law)
Kirchoff's Voltage Law
For every loop in an electrical circuit:
ΣiVi = 0
where Vi are all the voltages applied across the circuit.
Kirchoff's Current Law
At every node of an electrical circuit:
ΣiIi = 0
where Ii are all the currents flowing towards or away from the node in the circuit.
Physics Formulas: Electromagnetism
Here are some of the basic physics formulas from electromagnetism.
The coulombic force between two charges at rest is
| (F) = | q1q2 4πε0r2 |
- q1, q2 are charges
- ε0 is the permittivity of free space
- r is the distance between the two charges
The Lorentz force is the force exerted by an electric and/or magnetic field on a charged particle.
(Lorentz Force) F = q (E + v x B)
where
- q is the charge on the particle
- E and B are the electric and magnetic field vectors
Physics Fundamental Units
Fundamental units are those measurements which cannot be derived must must be measured.All other formulas can be derived from the fundemental units.
The fundamental units that are used for motion are:
| Quantity | Units | Symbol |
| length/distance | metres (m) | d |
| mass | kilogram (kg) | M |
| time | second (s) | T |
| temperature | degrees Kelvin (oK) | (theta) |
Velocity/Speed
Speed is the measurement of the movement of an object. Velocity is the speed plus direction.| Units | Symbol | Formula |
| meters per second (m/s) | v | v = d t |
Acceleration
Acceleration is the rate of change of speed. A negetive acceleration is commonly called deceleration.| Units | Symbol | Formula |
| meters per second squared (m/s2) | a | a = (v1 - v0) / t |
Distance
Distance travelled based on a speed/acceleration| Units | Symbol | Formula |
| meters (m) | d | d = v0 t + 1/2 a t2 |
Force
Something trying to move a mass.| Units | Definition | Symbol | Formula |
| Newtons (N) | 1 Newton of force is produced by acceleration of 1 kg of mass 1 m/s2 | F | force = mass * acceleration F = m a |
Weight
Weight, is not the same as mass. Weight is a measurement of the force of gravity on a mass. A mass for a given object is always the same no matter the force of gravity (e.g. the moon)| Units | Symbol | Formula |
| Newtons (N) | w | weight = mass * acceleration of gravity (9.8m/s2) |
Pressure
Force per unit areaClick here for info on tensile strength.
| Units | Definition | Symbol | Formula |
| Pascal (Pa) | 1 Pascal of pressure = 1 Newton of force applied over 1 square metre | ?? |
Work
A force moving a mass a distance.| Units | Definition | Symbol | Formula |
| Joules (J) kg m2/s2 Newton-meters (N m) | 1 Joule of work is produced by applying 1 Newton of force to move an object 1 metre | W | work = force * distance W = F d |
Energy
The ability (or capacity) to do work| Units | Definition | Symbol | Formula |
| Joules (J) Watt-hours Calories BTU | E | Ek = 1/2 m v2 |
Power
The rate at which energy is used.The rate at which work is done.
| Units | Definition | Symbol | Formula |
| Watts (W) | 1 Watt = 1 Joule per second 1 Watt = 1 Volt times 1 Ampere | P | P = V * I P = W / t |
Torque
A force that rotates an object around a point. A torque is not the same as work, although it is measure in the same units. The distance portion of the torque is the distance from the centre of rotation to the point of the force, unlike work, the distance is how far the force moves an object. To determine the amount of work a torque applies to its' point of rotation, you must multiple the torque by the distance the object is rotated through its' centre.| Units | Definition | Symbol | Formula |
| Newton-meters (Nm) | 1 Nm force is create by applying a force to a lever of 1 Newton, 1 metre from the point of rotation | T |
Rotational Work
The amount of work perfomed by a torque force rotating an object| Units | Definition | Symbol | Formula |
| Newton-meters (Nm) |
Power - Torque Relationship
Power and torque do have a relationship:Power = work / time Power = torque * RPM * (1min/60sec) * 2 * pi |
BIOLOGY
Cells are the Starting Point
 All living organisms on Earth are divided in pieces called cells. There are smaller pieces to cells that include proteins and organelles. There are also larger pieces called tissues and systems. Cells are small compartments that hold all of the biological equipment necessary to keep an organism alive and successful on Earth.
All living organisms on Earth are divided in pieces called cells. There are smaller pieces to cells that include proteins and organelles. There are also larger pieces called tissues and systems. Cells are small compartments that hold all of the biological equipment necessary to keep an organism alive and successful on Earth. A main purpose of a cell is to organize. Cells hold a variety of pieces and each cell has a different set of functions. It is easier for an organism to grow and survive when cells are present. If you were only made of one cell, you would only be able to grow to a certain size. You don't find single cells that are as large as a cow. Also, if you were only one cell you couldn't have a nervous system, no muscles for movement, and using the internet would be out of the question. The trillions of cells in your body make your life possible.
One Name, Many Types

There are many types of cells. In biology class, you will usually work with plant-like cells and animal-like cells. We say animal-like because an animal type of cell could be anything from a tiny microorganism to a nerve cell in your brain. Plant cells are easier to identify because they have a protective structure called a cell wall made of cellulose. Plants have the wall; animals do not. Plants also have organelles like the chloroplast (the things that make them green) or large water-filled vacuoles.

We said that there are many types of cells. Cells are unique to each type of organism. Humans may have hundreds of types of cells. Some cells are used to carry oxygen (O2) through the blood (red blood cells) and others might be specific to the heart. If you look at very simple organisms, you will discover cells that have no defined nucleus (prokaryotes) and other cells that have hundreds of nuclei (multinucleated). The thing they all have in common is that they are compartments surrounded by some type of membrane.
Looking at Cell Functions
 All cells have a purpose. If they don't do anything productive, they are not needed anymore. In the big picture, a cell's purpose is much more important than acting as small organizational pieces. They had their purpose long before they started working together in groups and building more advanced organisms. When alone, a cell's main purpose is to survive.
All cells have a purpose. If they don't do anything productive, they are not needed anymore. In the big picture, a cell's purpose is much more important than acting as small organizational pieces. They had their purpose long before they started working together in groups and building more advanced organisms. When alone, a cell's main purpose is to survive. Even if you were a single cell, you would have a purpose. You would have to survive. You would be moving around (probably in a liquid) and just trying to stay alive. You would have all of your pieces inside of you. If you were missing a piece you needed to survive, you would die. Scientists call those pieces organelles. Organelles are groups of complex molecules that help a cell survive.
All Cells are not Created Equal
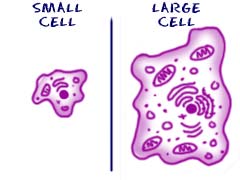
 In the same way that cells survive in different ways; all cells have different types and amounts of organelles. The larger a cell becomes the more organelles it will need. It makes sense if you think about it. If you are a big cell, you will need to eat more than a little cell. You will also need to convert that food into energy. A larger cell would need to eat more and may wind up having more mitochondria to process that food into energy.
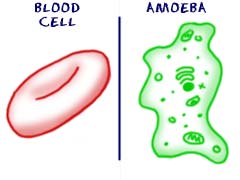
In the same way that cells survive in different ways; all cells have different types and amounts of organelles. The larger a cell becomes the more organelles it will need. It makes sense if you think about it. If you are a big cell, you will need to eat more than a little cell. You will also need to convert that food into energy. A larger cell would need to eat more and may wind up having more mitochondria to process that food into energy. While they might have a purpose, more advanced cells have a difficult time surviving on their own. A cell from your brain could not survive in a Petri dish for long. It doesn't have the right pieces to live on its own. It does have the ability to transmit electrical systems around your body. An amoeba could survive in a dish forever, thrive, and reproduce. On the other hand, that amoeba will never help you transmit electrical impulses. The brain cell is far more advanced and has specific abilities and organelles. Simpler cells have a better chance of surviving on their own while complex cells can accomplish tasks that are more advanced.
The Littlest Organisms
Let's study the wee ones of the world known as the microbes or the microorganisms. If you spend your life studying them, you would be a microbiologist. These are the smallest of the small and the simplest of the simple. Some of them, like viruses, may not even be alive as we currently define life.
What is a Microbe?
What makes a microbe? We suppose you need a microscope to see them. That's about it. There is a huge variety of creatures in this section. They can work alone or in colonies. They can help you or hurt you. Most important fact is that they make up the largest number of living organisms on the planet. It helps to be that small. It's not millions, billions, or trillions. There are trillions of trillions of trillions of microbes around the Earth. Maybe more.Calling all Microscopes
As with all of science, discovery in biology is a huge thing. While microbes like bacteria, fungi, some algae, and protozoa have always existed, scientists did not always know they were there. They may have seen a mushroom here or there, but there were hundreds of thousands of species to be discovered. It took one invention to change the way we see the world of microbes - the microscope. In 1673, Anton von Leeuwenhoek put a couple of lenses together and was able to see a completely new world. He made the first microscope. It wasn't that impressive, but it started a whole history of exploration. More important to us, scientists were eventually able to discover the cause and cure of many diseases.
It took one invention to change the way we see the world of microbes - the microscope. In 1673, Anton von Leeuwenhoek put a couple of lenses together and was able to see a completely new world. He made the first microscope. It wasn't that impressive, but it started a whole history of exploration. More important to us, scientists were eventually able to discover the cause and cure of many diseases. Too Many to Count, Too Small to Find
We'll give the big overview on the variety of microorganisms here. There is no simple explanation of a microbe besides the fact that they are small. The list goes on. Just remember that there is a lot of variety going on here.They can be heterotrophic or autotrophic. These two terms mean they either eat other things (hetero) or make food for themselves (auto). Think about it this way: plants are autotrophic and animals are heterotrophic.
They can be solitary or colonial. A protozoan like an amoeba might spend its whole life alone, cruising through the water. Others, like fungi, work together in colonies to help each other survive.
They can reproduce sexually or asexually. Sometimes the DNA of two microbes mixes and a new one is created (sexual reproduction). Sometimes a microbe splits into two identical pieces by itself (asexual reproduction).
Plant Basics
If you're not a microbe and you're not an animal, chances are you are a plant. There are loads of species of plants on Earth. Just as there is a system of classification for animals, there is also a system of classification for plants. Because plants adapt so well to any climate, scientists need a way to organize the hundreds of thousands of species.
What Makes a Plant?
What do they all have in common? The big thing that connects plants is photosynthesis. Photosynthesis is the process that allows plants to take energy from the Sun and create sugars. Not all plants go through the process of photosynthesis. As with all of biology, there are exceptions and you may learn about plant species that are parasites. Plants also have cell walls. In the cells tutorials we explained that all cells have a membrane. Only plants have an additional cell wall made from cellulose.Let's look at photosynthesis. Plants are able to turn sunlight into energy but not directly. Plants are actually able to store energy in some chemical bonds that can be used later. Before we get into details, we'll explain that there are two processes on Earth: Photosynthesis and Respiration. Photosynthesis stores the energy and respiration releases that energy. It all starts with the Sun. Check out the tutorial on photosynthesis.

Learning from Plants
Not only do you see plants everywhere in the real world, but they are also all over the scientific world. Scientists use them for studies in genetics. A guy named Gregor Mendel used pea pods and their flowers to come up with some of the first ideas on how traits are passed from one generation of organism to another (genetics). We also use plants for food. Scientists are constantly developing new plants that are more resistant to disease and insects. Scientists also help create plants that grow faster and make more food.INVERTEBRATES -
ONE OF TWO MAJOR ANIMAL GROUPINGS
There are two basic groups of higher animals. They are vertebrates and invertebrates. While both have advanced through the processes of evolution, there is one fundamental difference. Invertebrates do not have backbones. Both groups are in the Kingdom Animalia, but their bodies are organized differently. What makes invertebrates different? All invertebrates share common traits. At the bottom of the invertebrate world are the sponges. Sometimes they don't fit in but they are still part of the group. Here's the nice and neat little list. 
(1) They are multicellular. It's more than being a colony of individual cells. The cells are working together for the survival of the organism. All of the cells have specific duties and responsibilities.
(2) No backbone. We already talked about this one. That's the whole definition of invertebrate, no vertebrae.
(3) No cell walls. When we talked about plants, we always mentioned cell walls. Invertebrates don't have them. Remember that even if none of them look like animals, they are. Being an animal means you have no cell wall.
(4) Here are a few that have the qualifier "most" attached. That means not all of them have the trait, but most do. Most of them have tissues (not sponges) that are specific organizations of cells. Most of them reproduce sexually (not asexually). That means two gametes combine to form a new organism. Those gametes come from separate organisms (male and female).

Most invertebrates can move. Even sponges move when they are very young and very small. Once they settle down they don't move anymore. Other invertebrates like lobsters and insects move around their whole lives. Most invertebrates are organized in a way called symmetrical. Symmetrical organization means when you can draw a line down the middle of the organism and the two sides look like mirror images. Draw a line down the middle of yourself and one side looks like the other side. If you draw a line down the middle of an octopus you would find two sides with equal parts. Remember we said most? Sponges and some coral are not symmetrical.
(5) Invertebrates can't make their own food. Scientists use the word heterotrophic. Heterotrophs feed off other things to get their energy. Plants are autotrophic. They make their own food. Being heterotrophic is one of the main characteristics of being an animal. We eat things, whether it is plants or other animals. That's just the way the world works.
VERTEBRATE BASICS
Vertebrates are the most advanced organisms on Earth. The traits that make all of the animals in this section special are their spinal cords, vertebrae, and notochords. It's all about having a series of nerves along your back (dorsal side). If you are an organism, you can't just have the nerves sitting there. You need to give those nerves support and protection. That need brings us to the backbones and a rod of cartilage called the notochord.
NOT SO MANY SPECIES
Fifty thousand species might seem like a lot. Compared to the invertebrates, there are not that many species of vertebrates. You might be asking why. One reason is that vertebrates are usually larger than invertebrates. They need more space. Another reason is that, even though they are more advanced, there are many limitations on the environments that are available to them.Think about it this way. If you are smart mammal, would you rather live near the ocean or in the frozen tundra of the arctic? Many land animals can make that decision and move to more desirable areas for living. Those nicer areas can only support so many species of animals.

THEY'VE GOT THE BRAINS
Vertebrates are smart. Some of them are very smart. We mean you. Most vertebrates have very advanced nervous systems. While a goldfish might not compare to your intelligence, when you compare a goldfish to a sea anemone, a goldfish is like Einstein. Octopi are probably the smartest invertebrates and may equal or be smarter than some vertebrates. Octopi are the exception in the invertebrate category.More cool traits about vertebrates are that they have muscles and skeletons. While the materials may vary, muscles allow vertebrates to move around very efficiently and perform complex moves. That ability to move and the intelligence to go with it gives vertebrates such as reptiles and birds an advantage in the natural world.
What is a System?
 A system is a group of organs that work together and provide an organism with an advantage for survival. It is the most complex organization in your body and the final level of the progression from cells to tissues to organs and then systems. Systems work alone and with other systems to allow your body to maintain homeostasis. Homeostasis is a stable internal environment that allows you (and your cells) to survive.
A system is a group of organs that work together and provide an organism with an advantage for survival. It is the most complex organization in your body and the final level of the progression from cells to tissues to organs and then systems. Systems work alone and with other systems to allow your body to maintain homeostasis. Homeostasis is a stable internal environment that allows you (and your cells) to survive. While every one of your systems is needed to survive, your nervous system is the most important as you continue reading this page. Your eyes and brain are reading these words and remembering all of the information about systems. If you think about it, you are also using your muscular system to help move your eyes, pupils, and keep your head up.
Organs Working Together
Organs are a part of every system. Your heart is classified as an organ and it is a part of the circulatory system. Organs can work within several systems of your body. Many organs also have specific cells or tissues that have different functionality. Your kidneys are not only a part of your excretory system; they also have specific parts that serve the endocrine system.You, and many advanced mammals, have similar organs and systems. However, there is a wide variety of organ types found throughout the animal kingdom. Some aquatic animals have organs that remove salts from salt water and an animal like a cow might have multiple stomachs in the digestive system.
Systems Can't Work Alone
 We just explained how organs could be a part of several systems. Similarly, systems rarely work alone. All of the systems in an organism are interconnected. A simple example is the connection between the circulatory and respiratory systems. As blood circulates through your body, it eventually needs fresh oxygen (O2) from the air. When the blood reaches the lungs, part of the respiratory system, the blood is re-oxygenated. Your stomach, part of the digestive system, constantly interacts with your endocrine system and spreads hormones throughout your body.
We just explained how organs could be a part of several systems. Similarly, systems rarely work alone. All of the systems in an organism are interconnected. A simple example is the connection between the circulatory and respiratory systems. As blood circulates through your body, it eventually needs fresh oxygen (O2) from the air. When the blood reaches the lungs, part of the respiratory system, the blood is re-oxygenated. Your stomach, part of the digestive system, constantly interacts with your endocrine system and spreads hormones throughout your body. Examples of Systems
It's easy to point out a few in your body. The two you think of the most are probably your respiratory and digestive system. A couple of times a day you might get hungry, sit down, and have a nice meal. All of that food gets broken down in your digestive system so that your body has energy to survive. The stuff your body doesn't need is pooped out at the other end.Since you're breathing all of the time, the respiratory system is always at work. You breathe in and out from your nose and mouth while your lungs are the main organs that allow your body to absorb the oxygen you need from the air. There are many other systems in your body and specialized systems in other animals around the world.



